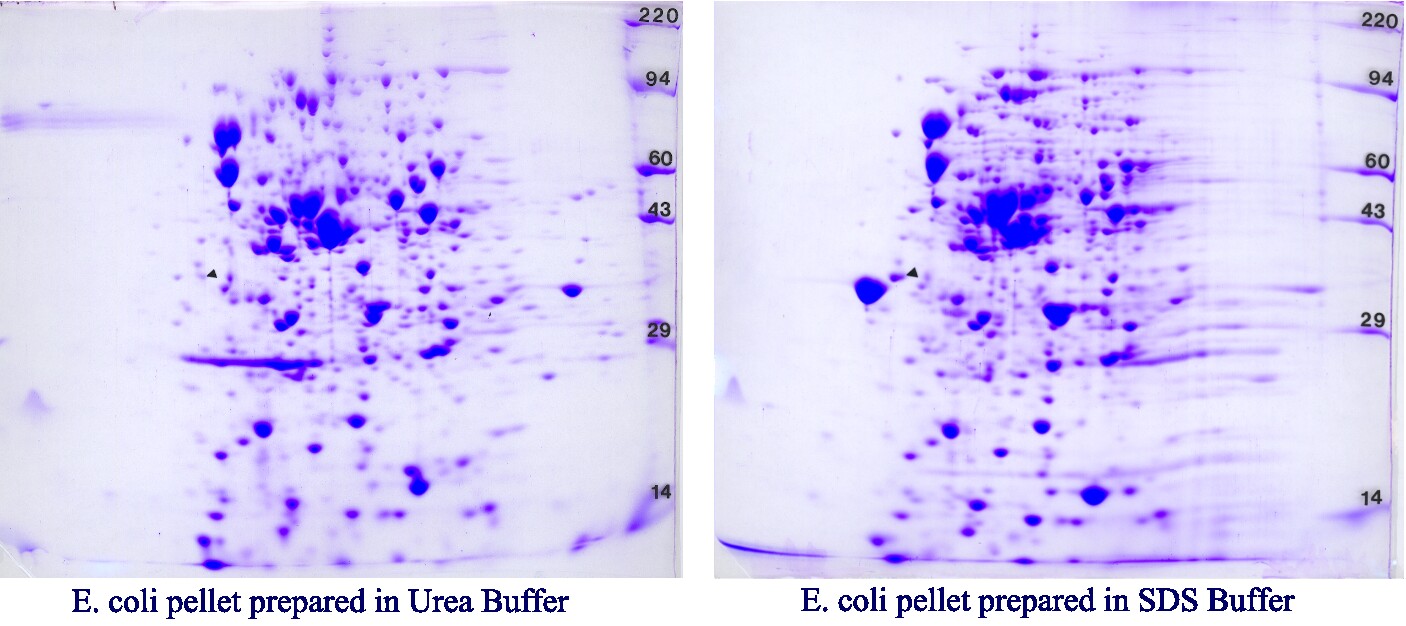
Host Cell Proteins (HCPs) play a crucial role in biologic product development, impacting product safety, efficacy, and overall quality. Ensuring comprehensive coverage and effective testing of HCP antibodies is essential for evaluating the purity and consistency of biologic products. In this article, we delve into the significance of HCP antibody coverage, explore Kendrick Labs’ specialized approach to HCP antibody testing, discuss key considerations for evaluating coverage, and highlight the impact of robust testing on biologic product quality.
Defining Host Cell Protein (HCP) Antibody Coverage
Host Cell Proteins (HCPs) are proteins derived from the host cells used to produce biologic products. Antibody coverage refers to the ability of antibodies to specifically detect and quantify these HCPs in biologic samples.
Significance of HCP Antibody Coverage in Biologic Product Testing
Accurate assessment of HCP levels is crucial for evaluating the purity, safety, and efficacy of biologic products, as HCP impurities can impact product quality and patient safety.
Importance of HCP Antibody Testing in Biologic Product Development
HCPs can potentially trigger immune responses in patients, affecting the safety and efficacy of biologic products. Testing for HCP antibodies helps ensure that these proteins are monitored and controlled during product development.
Challenges Posed by HCPs in Biologic manufacturing
The presence of HCP impurities poses challenges in biologic manufacturing, requiring rigorous testing and monitoring to meet regulatory standards and ensure product quality.
Overview of Kendrick Labs’ Approach to HCP Antibody Testing
Kendrick Labs employs cutting-edge techniques and advanced technologies to develop customized HCP antibody testing solutions tailored to the specific needs of each biologic product.
Advantages of Kendrick Labs’ Customized HCP Antibody Solutions
By offering personalized HCP antibody testing services, Kendrick Labs provides comprehensive and accurate analysis to support biologic product developers in meeting regulatory requirements and ensuring product quality.
Key Considerations for Evaluating HCP Antibody Coverage
When choosing HCP antibody testing services, factors such as sensitivity, specificity, reproducibility, and customization capabilities are crucial in ensuring accurate and reliable results.
Interpreting and Utilizing HCP Antibody Coverage Data Effectively
Understanding and effectively utilizing HCP antibody coverage data can help biologic product developers make informed decisions, optimize product quality, and streamline regulatory compliance processes.
Impact of Comprehensive HCP Antibody Testing on Biologic Product Quality
When it comes to biologic product testing, the role of Host Cell Protein (HCP) antibody testing cannot be overstated. Kendrick Labs is at the forefront of enhancing product purity and safety through robust HCP antibody testing. By ensuring thorough testing for HCP antibodies, biologic products can meet stringent quality standards, safeguarding patient health and minimizing risks associated with impurities.
Regulatory Compliance and Quality Assurance Benefits of Comprehensive HCP Antibody Testing
Comprehensive HCP antibody testing not only enhances product quality but also plays a crucial role in maintaining regulatory compliance and ensuring quality assurance. Kendrick Labs’ advanced testing methodologies provide biopharmaceutical companies with the confidence that their products meet regulatory requirements and adhere to industry best practices. This level of assurance is invaluable in a highly regulated industry where adherence to standards is non-negotiable.
Demonstrating the Efficacy of Kendrick Labs’ HCP Antibody Testing
Real-world applications and success stories underscore the efficacy of Kendrick Labs’ HCP antibody testing services. Through implemented HCP antibody testing strategies, biopharmaceutical companies have gained valuable insights into the quality and purity of their products. These case studies highlight the tangible benefits of partnering with Kendrick Labs in ensuring the safety and efficacy of biologic products.
Results and Insights from Implemented HCP Antibody Testing Strategies
The results and insights derived from implemented HCP antibody testing strategies offer valuable learnings for the biopharmaceutical industry. By leveraging Kendrick Labs’ expertise in HCP antibody testing, companies can optimize their product development processes, mitigate risks, and enhance overall product quality. These insights pave the way for continued advancements in biologic product testing methodologies.
Future Trends in HCP Antibody Coverage for Biologic Product Testing
Innovations and evolutions in HCP antibody testing technologies are shaping the future of biologic product testing. Kendrick Labs remains at the forefront of these advancements, driving continuous improvement in testing methodologies to meet the evolving needs of the industry. Anticipated developments in regulatory guidelines and industry standards underscore the importance of staying ahead of the curve in HCP antibody coverage for biologic product testing.
In conclusion, a thorough understanding of HCP antibody coverage is paramount in the realm of biologic product testing. By embracing advanced testing methodologies and partnering with experienced laboratories like Kendrick Labs, Inc, biopharmaceutical companies can enhance the safety, quality, and compliance of their products. As the industry continues to evolve, staying abreast of emerging trends in HCP antibody testing will be crucial for driving innovation and ensuring the efficacy of biologic therapies in the future.